
If you manage a course in Arizona, California, Colorado, Florida, South Carolina, New Mexico, or Texas and your water report shows high pH and alkalinity, you probably see the same pattern on the ground. Scale builds on heads and nozzles. Fairways never quite flush. Iron and wetting agents help for a few weeks, then the same chlorosis and dry spots can return.
In many of these cases, the real problem sits in one line on the report: bicarbonates.
Bicarbonate (HCO₃⁻) is common in well water, Colorado River water, blends, and reclaimed water across the Southwest and Sunbelt. It drives alkalinity and shapes how irrigation water behaves once it moves into the soil.
When bicarbonate and carbonate are high and calcium and magnesium are low, irrigation water encourages calcium carbonate scale. Calcium and magnesium fall out as carbonates. Sodium stays in solution and stays in the soil exchange complex. Soil pH trends higher and sodium hazard rises.
On a golf course or sports field, that often looks like:
Those deposits signal bicarbonate and alkalinity sit out of balance with calcium, magnesium, and sodium in your irrigation water.
Soil pH shapes nutrient availability and how salts interact in the rootzone. For most turfgrass systems, water in a pH range close to 6–7 and soil pH around 6.5 supports better availability of both macronutrients and micronutrients. Penn State Ag Sciences
As soil pH climbs above that range, phosphorus and micronutrients such as iron, manganese, and zinc bind more strongly to soil particles and become harder for roots to take up. On warm-season turf and overseeded rye in AZ, CA, FL, NM, and TX, that often shows up as:
When bicarbonate also pushes sodium upward, calcium and magnesium no longer hold soil structure as well. Surfaces soften, infiltration slows, and leaching salts out of the profile takes more water and more care. It can feel futile when the water you’re using to leach is the same high-sodium water that is causing the issues.
With long seasons, high evapotranspiration, and limited leaching rain in some areas, that chemistry stacks up over time.
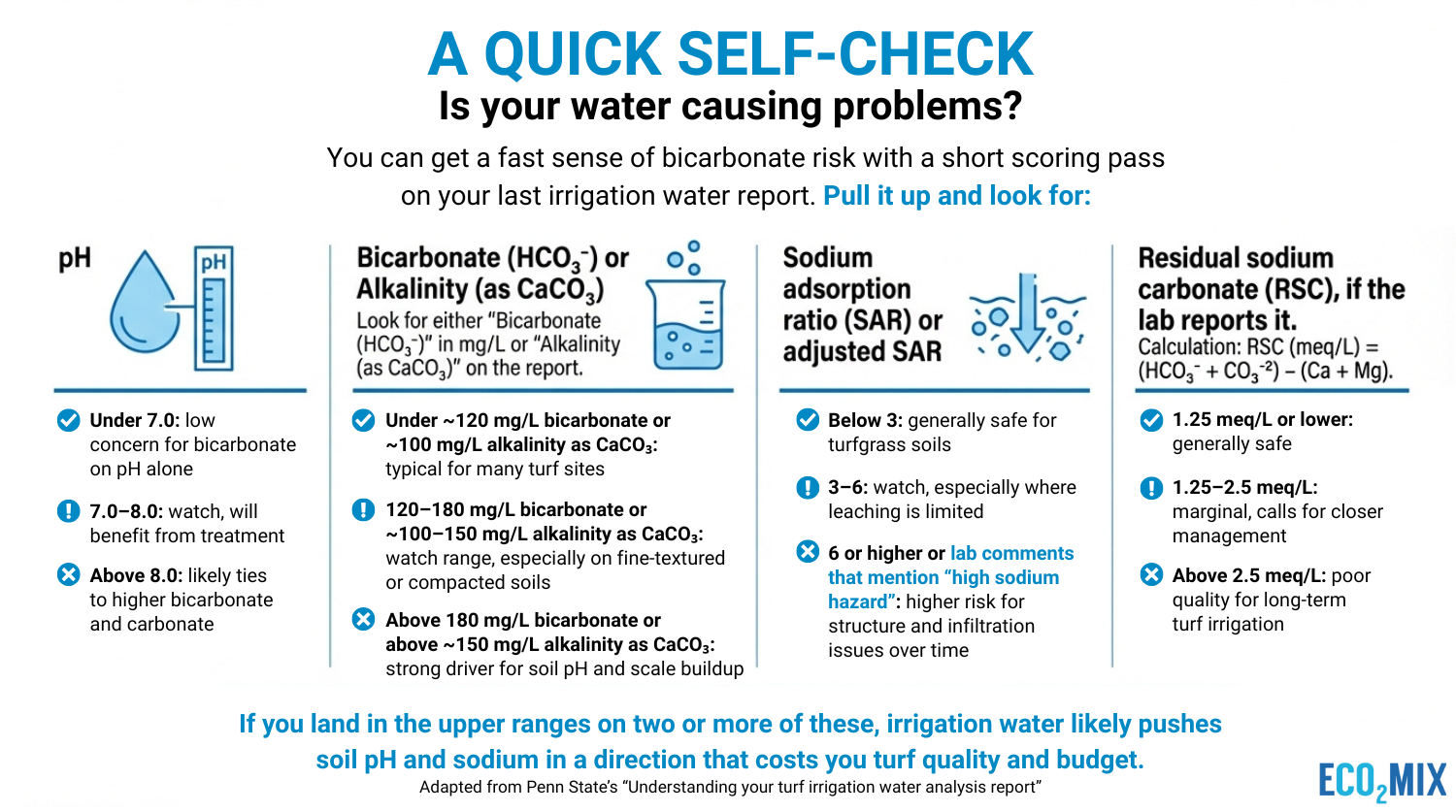
You can get a fast sense of bicarbonate risk with a short scoring pass on your last irrigation water report. Pull it up and look for:
If you land in the upper ranges on two or more of these, irrigation water likely pushes soil pH and sodium in a direction that costs you turf quality and budget. For a deeper walkthrough of how to read these numbers, you can review Penn State’s “Understanding your turf irrigation water analysis report,” which lays out the main parameters and guideline ranges in more detail.
Testing still matters, but you also need a plan once you know you face high bicarbonate.
A practical plan often includes:
Water acidification plays a central role in that last piece. When you lower water pH and neutralize bicarbonate at the pump station, you keep more calcium and magnesium in solution, reduce scale, and slow the rise of sodium hazard.
Strong mineral acids such as sulfuric and hydrochloric can do that chemistry, but they bring handling risk, corrosion, and extra sulfate or chloride load that many soils do not need. That is where carbonic acid offers another path. (Comparing Common Methods of Water pH Control)
Carbonic acid gives superintendents in high-alkalinity regions another option for pH control. ECO2MIX dissolves CO₂ into irrigation water at the pump station. That CO₂ forms carbonic acid, sometimes also referred to as Dissolved CO2 in Irrigation Water (DCIW), which lowers water pH into a target range and neutralizes bicarbonate before the water reaches the sprinklers.
For courses, this approach:
Courses that move to carbonic acid pH control report healthier turf with better color, and fertilizer savings, and fewer chronic pH-related turf problems when water treatment lines up with solid fertility and cultural practices. The CO₂ added to the soil also improves soil health and microbial activity.
If bicarbonate and high pH limit what you can get from your irrigation water, or you’re exploring alternative acids for pH control, the next step is a short call to see whether ECO2MIX fits your operation.
In a consultation, we will:
If you want to explore that fit, you can schedule a superintendent consultation here: