Managing water quality on a golf course sits at the crossroads of science, strategy, and whatever your water source decides to do that season. As irrigation water becomes tougher to manage in many regions, turf managers are looking at new tools that help keep pH in a workable range, protect systems, and avoid long term damage to soil.
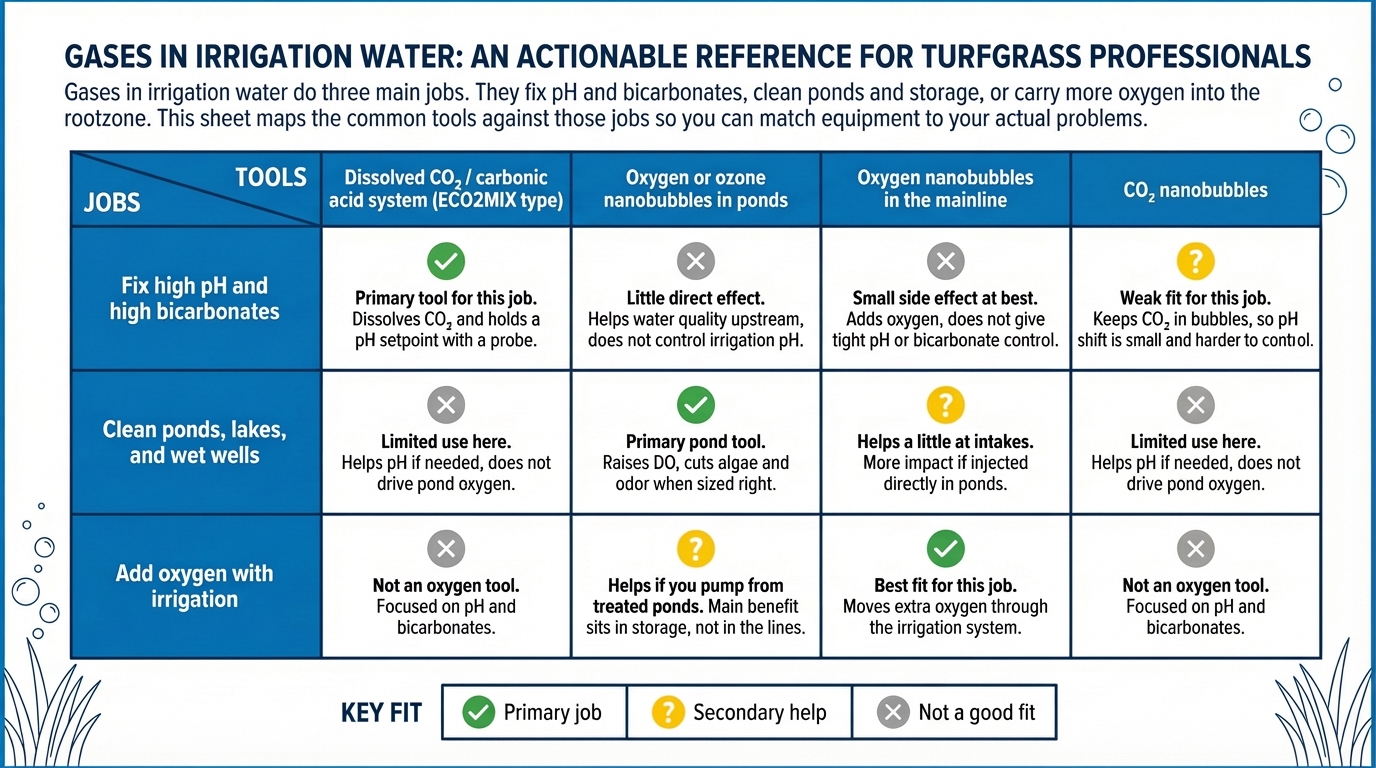
Two kinds of systems get grouped together a lot right now: nanobubbles and carbonic acid pH control. They both involve adding gas to water, so they sound similar. On a golf course they do very different jobs. One focuses on oxygen and biology. The other focuses on pH and bicarbonates.
ECO2MIX supports superintendents who want safer, smarter, and more sustainable water programs. This guide explains what each technology actually does and why dissolved CO₂ (carbonic acid) has become the preferred option for accurate pH control, especially on high pH or recycled water.
Can CO₂ nanobubbles control irrigation pH?
They can nudge pH a little, but they are not a good primary pH control tool. Reliable pH and bicarbonate control comes from CO₂ that is fully dissolved and injected to reach a target pH measured by a probe at the pump.
Are CO₂ nanobubbles the same as carbonic acid or ECO2MIX?
No. CO₂ nanobubbles keep gas in bubbles for oxygen and biology work. Carbonic acid comes from dissolved CO₂ that actually shifts pH and bicarbonates. ECO2MIX is a dissolved CO₂ system that does that pH job in the mainline.
Are oxygen or ozone nanobubbles a good choice for ponds?
Yes. That is one of the jobs where they do very well. They raise dissolved oxygen, help reduce algae and odor, and improve storage water quality when they are sized and run properly.
Are ultrafine or nanobubble systems really “many times better than rainwater”?
Ultrafine and nanobubble systems can push dissolved oxygen in ponds and wet wells much higher than untreated storage and often higher than typical rainwater. That matters for algae control, muck reduction, and pond biology. It is. different job than carbonic acid or pH and bicarbonate control. High dissolved oxygen helps biology and water quality.
What does dissolved CO₂ or carbonic acid do in irrigation water?
It lowers irrigation water pH into a turf-friendly range and neutralizes bicarbonates that drive scale and high soil pH. It does this without loading the system with sulfate or chloride the way strong mineral acids do.
Can one system handle ponds, oxygen, and pH control?
Usually not. Nanobubble systems and dissolved CO₂ systems are built for different mechanical goals, so they do different jobs. Many courses that use gas tools pair a pH system on the mainline with oxygen tools in ponds or tanks if they still need them.
On most courses gases in water show up in three ways.
Here equipment injects very small bubbles into lakes, wet wells, or storage ponds to raise dissolved oxygen, cut algae, reduce odor, and improve water quality at the intake.
In this role the goal is extra oxygen in the irrigation stream. That can support soil aeration in some situations and can help with biofilm or clogging in lines and emitters.
This is the pH and bicarbonate job. CO₂ fully dissolves into the mainline, forms carbonic acid, and pulls pH and alkalinity into a tighter range before water reaches turf. For this to happen, there should not be any bubbles of CO₂.
Those are three separate jobs. Some courses need all three. Some need none. Matching each tool to the job it actually does matters more than the label.
Water does not care whether CO₂ came from a cylinder, a nanobubble generator, or the air. Once it dissolves, the chemistry looks the same.
You can think of it in three steps:
Hydrogen ions lower pH. Bicarbonate carries most of the alkalinity. Labs often report alkalinity as “ppm as CaCO₃” but in many irrigation waters the main species behind that number is HCO₃⁻.
For turf irrigation water, Penn State and others describe these ranges as common targets or flags:
You can see a full table of these limits in Penn State’s irrigation water guidelines for turfgrass sites:
https://extension.psu.edu/irrigation-water-quality-guidelines-for-turfgrass-sites
A dissolved CO₂ system for pH control, often called DCIW (Dissolved CO₂ in Irrigation Water), pushes CO₂ through this whole chain inside a reactor at the pump, then injects that into the mainline. Gas dissolves, becomes carbonic acid, neutralizes bicarbonate, and delivers water that hits the pH setpoint before it leaves the pump station.
CO₂ nanobubbles only join this chain when CO₂ escapes from the bubbles and dissolves. That difference sits at the center of how these tools behave.
Nanobubble generators and dissolved CO₂ systems are not the same machine with different settings. They are built for different outcomes.
A dissolved CO₂ system for pH control has one main mechanical goal. It wants dissolved gas. It holds CO₂ and water together under the right pressure and mixing so gas crosses the gas–water boundary quickly and completely. A pH probe watches the treated water, and the system adjusts the CO₂ feed to hold a setpoint.
A nanobubble system has a different mechanical goal. It wants bubbles that stay bubbles. It uses pressure changes, shear, and special nozzles to create huge numbers of very small bubbles and slow their rise and escape. In ponds and tanks that is what you want. A cloud of persistent bubbles spreads oxygen through the water column and through biofilm.
Those design choices mean the equipment is not the same:
When CO₂ rides along in nanobubbles and only dissolves later and in patches, carbonic acid often forms in the wrong place or at the wrong time for mainline pH control. That is why CO₂ nanobubbles are a weak fit for that particular job, even though nanobubbles are useful for other jobs.
In ponds and reservoirs oxygen or ozone nanobubbles are a strong option.
They raise dissolved oxygen even at depth, support aerobic microbes, and help oxidize organic sludge and reduced metals. Case studies and independent articles on golf lakes describe higher dissolved oxygen through the water column, fewer nuisance blooms, and cleaner intakes when these systems run correctly (SOLitude case study on a Florida golf community lake).
The pond job looks like this:
This work sits upstream of the pump station. Nanobubble systems in ponds aim at oxygen and appearance, not at a specific irrigation pH. They are a solid tool when ponds and tanks cause the most trouble.
Some systems also use ozone nanobubbles in ponds or wet wells to oxidize pathogens in the water column and help reduce disease pressure that starts from irrigation water. That fits the same oxygen and oxidation job very well.
For irrigation, nanobubble systems can inject oxygen nanobubbles into the irrigation mainline. In that role the promise shifts from pond cleanup to extra oxygen in the rootzone and in lines.
Turf and field research gives a mixed but useful picture.
In lines, micro and nanobubble water can slow emitter clogging by pulling particles, shifting biofilm structure, and lowering scaling in drip systems. Vendors sometimes report reductions in wetting agents, fertilizer, or fungicide use when oxygen nanobubbles improve pond health or water movement in soils. Those benefits sit on the biological and oxygen side of the program. They do not change the basic point that oxygen tools and pH tools do different jobs in the system.
For turf irrigation this leads to a clear picture:
When CO₂ nanobubbles are sold for mainline pH control, the chemistry still depends on dissolved CO₂. The nanobubbles act as a transport stage. Some of the gas dissolves and does the pH work. The rest remains in bubbles longer. That pattern increases CO₂ use and weakens control at the pump compared with a DCIW system that forces dissolution in a contact chamber and uses a pH probe.
Nanobubbles fit the oxygen job. CO₂ nanobubbles do not replace a purpose-built pH system.
Dissolved CO₂, or carbonic acid in irrigation water, begins with CO₂ dissolved in water in a reactor. The aim is to dissolve the gas and create water that behaves, in acidity, like rainwater, but held in a controlled range.
Rainwater picks up CO₂ from the air, forms carbonic acid, and reaches the ground with a mildly acidic pH. Turf does not need irrigation water as acidic as fresh rain, which is around 5.5 pH, especially on sand-based rootzones. But the same chemistry helps when source water pH starts high.
In turf irrigation water:
Carbonic acid helps in three main ways.
When less bicarbonate pairs with calcium, more of that calcium stays in solution in the soil water, where it can support structure, displace sodium, and actually reach the plant.
Peer-reviewed work in calcareous vineyards has shown that carbonated irrigation can lower soil pH in the rootzone and improve nutrient uptake, chlorophyll, and yield in stressed grapevines. One example is Lampreave et al. in Agriculture (2022):
https://doi.org/10.3390/agriculture12060792
Turf-specific work on dissolved CO₂ and carbonic acid irrigation is underway, including trials with ECO2MIX irrigation water at the University of Florida Fort Lauderdale Research and Education Center with Dr. Marco Schiavon that began in 2025.
For courses dealing with high pH and high bicarbonate water, moving irrigation pH into the 6.0 to 7.0 range with dissolved CO₂ cuts bicarbonate, reduces scale, and supports better nutrient use and has soil health benefits. That is the core of the pH control job.
Recycled water often brings a reliable supply and a tougher chemistry profile in the same pipe. Water reports commonly show higher levels of salts, sodium, and bicarbonates. These can damage soil structure and stress turf when they accumulate.
USGA guidance on recycled water use highlights these issues and recommends careful water and soil testing with targeted maintenance to manage them. Useful starting points include:
On these sites, a dissolved CO₂ system for pH and bicarbonate control is often the first lever to pull:
If ponds and storage still struggle with oxygen and algae after that, oxygen nanobubbles can sit on top as a separate tool. The chemistry base still starts with pH and bicarbonates.
ECO2MIX sits in the dissolved CO₂ lane and focuses on the pH and bicarbonate job.
For a superintendent several points matter:
Courses that shift from sulfuric acid or burned sulfur systems to carbonic acid pH control see a practical move toward zero corrosion issues in their irrigation network while they still clear scale and plugging. ECO2MIX delivers that carbonic acid approach in a way that lines up with turf irrigation water guidelines and uses less CO₂ than less efficient delivery methods.
Some ultrafine or nanobubble systems now offer carbonic acid as an add-on for pH control. In those setups you still judge the pH job by the same questions:
The nanobubble part still does the oxygen and pond biology work. The carbonic acid part still needs to behave like a true dissolved CO₂ / DCIW system if you want reliable pH and alkalinity control.
For pH and bicarbonate control in irrigation water three questions matter:
A DCIW system such as ECO2MIX:
A CO₂ nanobubble system that tries to handle pH:
In both cases the acid in play is carbonic acid. The difference sits in delivery and in control.
For accurate pH control in an irrigation system, the CO₂ that dissolves in the contact chamber and sits under a pH probe is the gas doing the real work. Gas that remains in bubbles and dissolves late works against you on that job.
Nanobubble delivery fits oxygen and pond jobs. Direct dissolved CO₂ fits the pH and bicarbonate job. Seeing them as separate jobs makes it easier to sort through vendor claims, read your water report, and decide which equipment belongs on your course.
If you want a deeper look at how irrigation water pH affects turf health, soil structure, and system performance, you can also watch the full ECO2MIX webinar replay.
You will see:
It is a useful follow up if you want to connect this guide to real on-course examples and see how dissolved CO₂ behaves in practice.